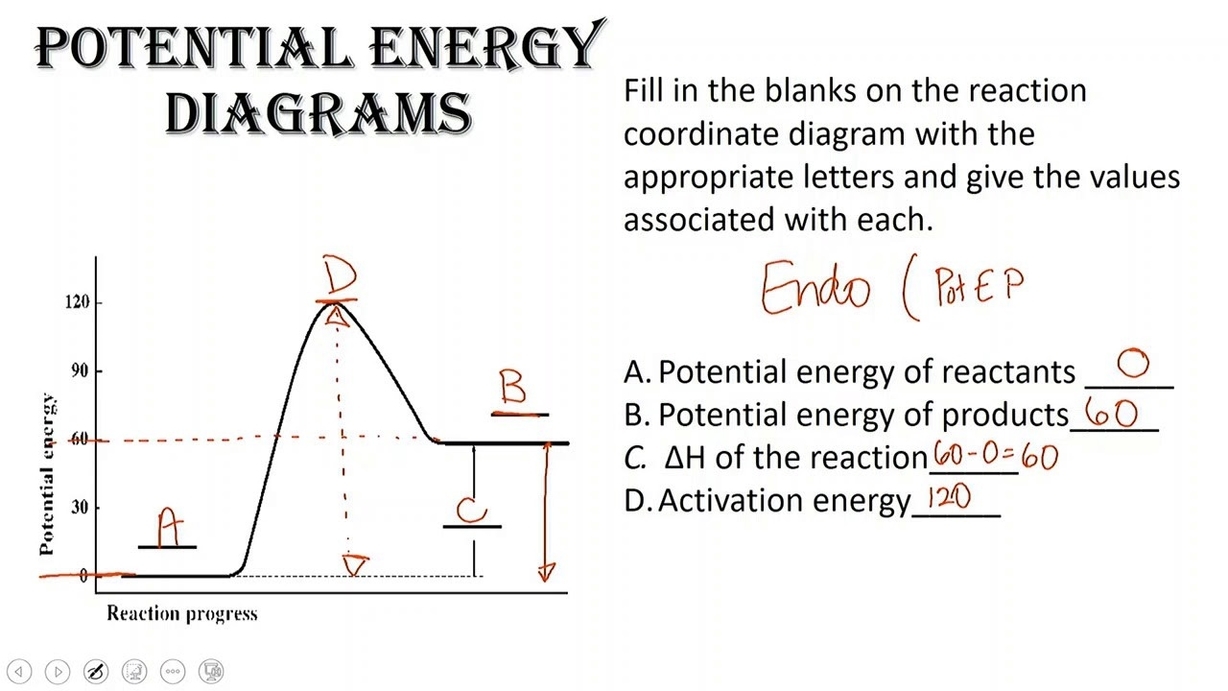
Energy is a fundamental concept in physics that describes the capacity for doing work or producing heat. Energy can exist in various forms, such as potential, kinetic, thermal, electrical, chemical, nuclear, and so on. Energy can also be transferred from one object or system to another, or converted from one form to another. However, energy can never be created or destroyed; this is the principle of conservation of energy.
Potential energy is the energy stored in an object or system due to its position or configuration. For example, a stretched spring has potential energy because it can release its tension and do work. Similarly, a book on a shelf has potential energy because it can fall down and do work. The amount of potential energy depends on factors such as mass, height, distance, or elasticity.
Kinetic energy is the energy of motion. Any object that is moving has kinetic energy. For example, a bullet fired from a gun has kinetic energy because it can hit a target and do work. Similarly, a car driving on a road has kinetic energy because it can move other objects and do work. The amount of kinetic energy depends on factors such as mass and speed.
Thermal energy is the energy of heat. It is related to the random motion and vibration of the molecules in a substance. The faster the molecules move, the higher the temperature and the thermal energy. For example, a burning candle has thermal energy because it can melt wax and do work. Similarly, a cup of hot coffee has thermal energy because it can warm up a person and do work. The amount of thermal energy depends on factors such as mass, temperature, and specific heat capacity.
Electrical energy is the energy of electric charges. It is related to the movement or flow of electrons in a circuit. The faster the electrons move, the higher the current and the electrical energy. For example, a battery has electrical energy because it can power a device and do work. Similarly, a lightning bolt has electrical energy because it can strike a tree and do work. The amount of electrical energy depends on factors such as voltage, current, and resistance.
Chemical energy is the energy stored in the bonds between atoms and molecules. It is related to the rearrangement of atoms and molecules in a chemical reaction. The breaking and forming of bonds releases or absorbs energy. For example, a match has chemical energy because it can ignite and do work. Similarly, a glucose molecule has chemical energy because it can be broken down and do work. The amount of chemical energy depends on factors such as the type and amount of reactants and products.
Nuclear energy
