Here is an essay about the structure of atom in approximately 1000 words:
“`
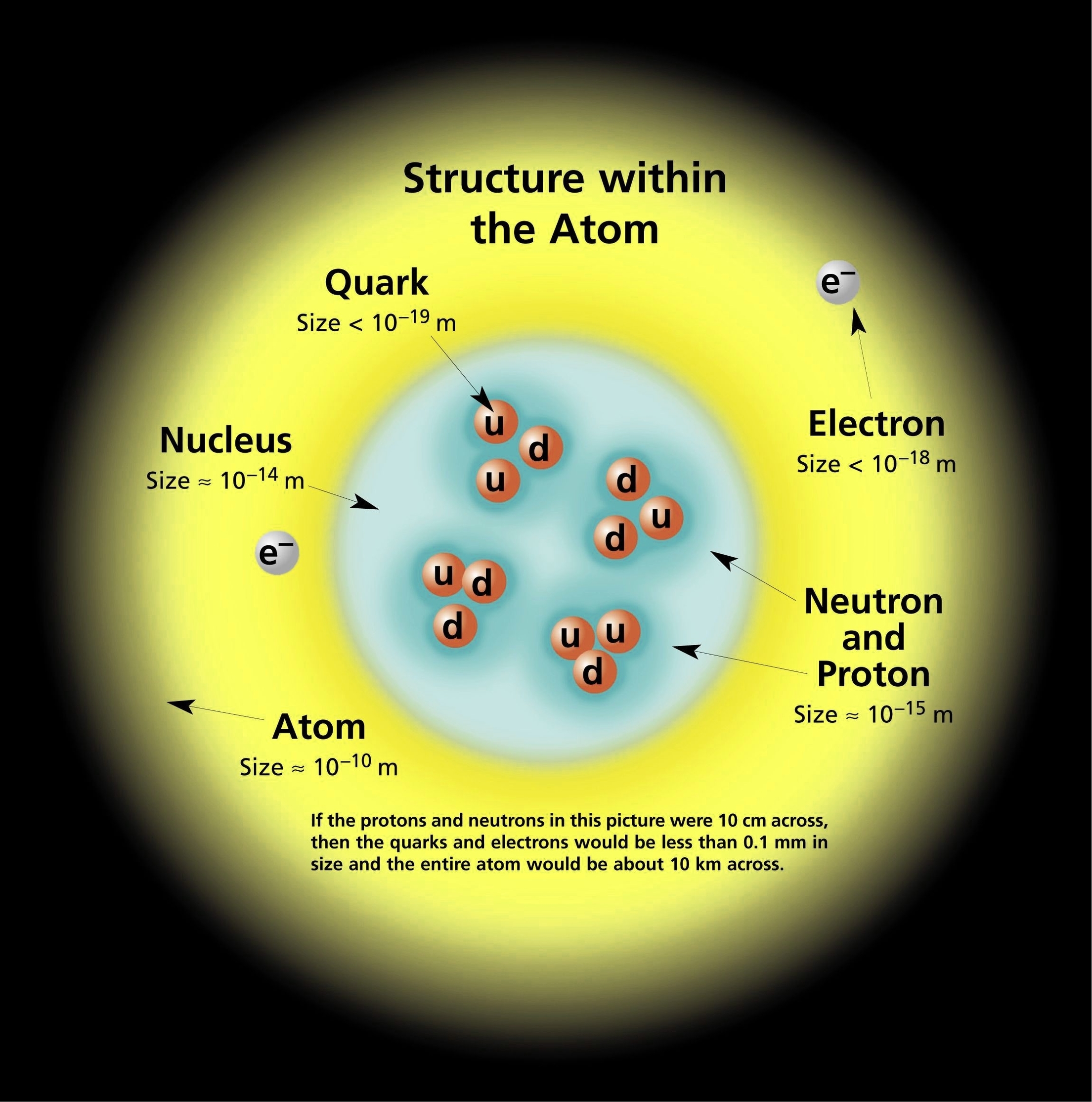
The structure of atom is one of the most fundamental topics in chemistry. An atom is the smallest unit of matter that retains the identity of a chemical element. Atoms are composed of three types of subatomic particles: protons, neutrons, and electrons. In this essay, we will explore the history, properties, and models of atoms, as well as their interactions and applications.
The concept of atom dates back to ancient times, when philosophers such as Democritus and Leucippus proposed that matter is made of indivisible and indestructible particles. However, it was not until the 19th century that scientists began to experimentally investigate the nature of atoms. John Dalton proposed the atomic theory, which states that all matter is composed of atoms of different elements, and that atoms of the same element have the same mass and properties. J.J. Thomson discovered the electron, the first subatomic particle, by observing the deflection of cathode rays in a magnetic field. He proposed the plum pudding model, which depicts the atom as a sphere of positive charge with embedded electrons. Ernest Rutherford disproved this model by performing the gold foil experiment, in which he bombarded a thin sheet of gold with alpha particles and observed their scattering. He concluded that the atom has a tiny and dense nucleus, which contains most of the mass and positive charge of the
