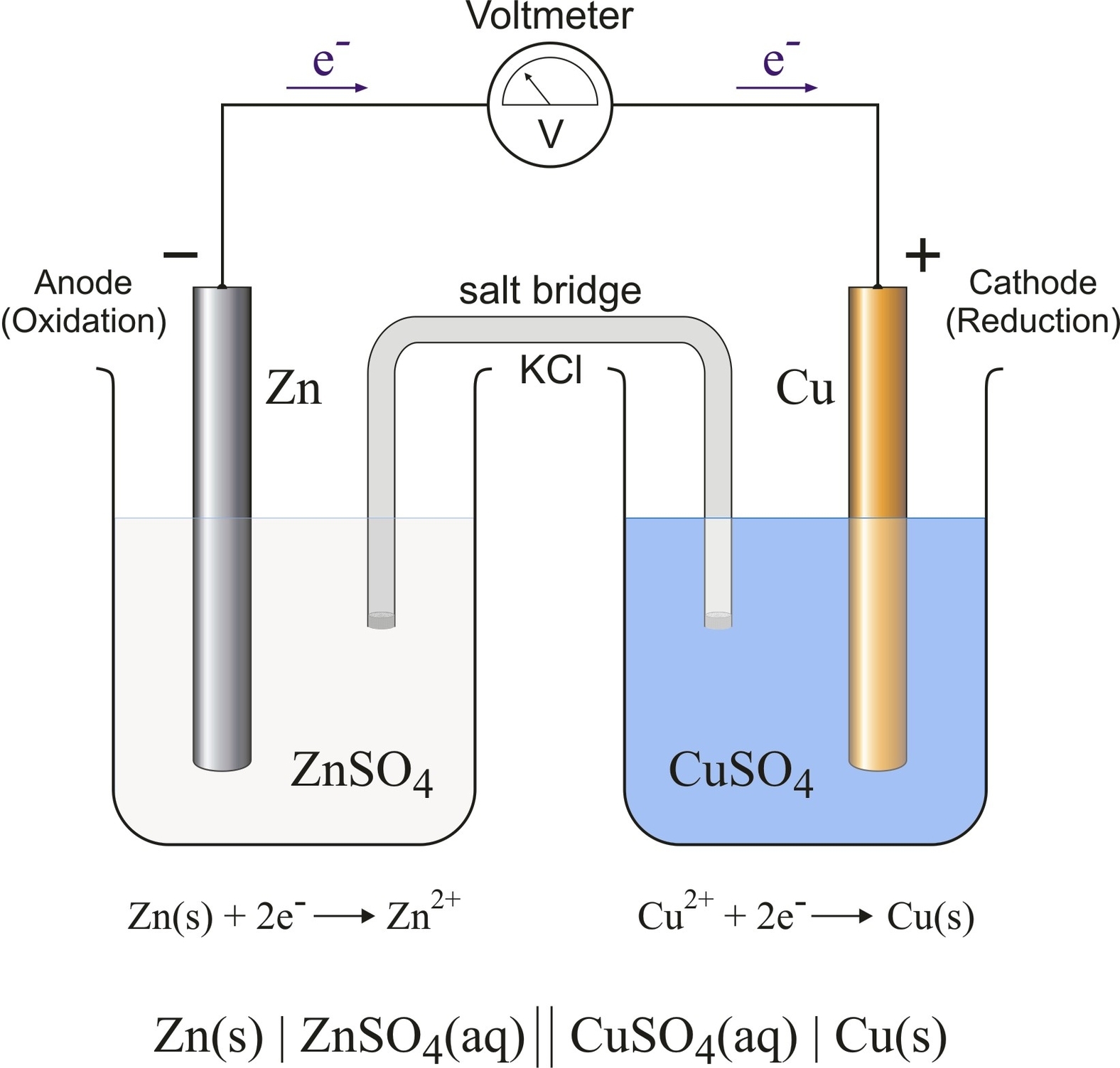
A galvanic cell is a device that converts chemical energy into electrical energy by using spontaneous redox reactions. It consists of two electrodes, each immersed in an electrolyte solution that contains ions of the same metal as the electrode. The electrodes are connected by a wire and a salt bridge or a porous membrane that allows the flow of ions but prevents the mixing of the solutions.
The electrode where oxidation occurs is called the anode, and the electrode where reduction occurs is called the cathode. The anode loses electrons and the cathode gains electrons, creating an electric potential difference between them. This potential difference drives the flow of electrons through the external circuit, generating an electric current. The magnitude of the potential difference depends on the nature of the electrodes and the electrolytes, and can be calculated using the Nernst equation or a standard reduction potential table.
A common example of a galvanic cell is the Daniell cell, which uses zinc and copper electrodes and their respective sulfate solutions as electrolytes. The half-reactions and the overall reaction for the Daniell cell are:
$$text{Anode: } text{Zn}(s) rightarrow text{Zn}^{2+}(aq) + 2text{e}^-$$
$$text{Cathode: } text{Cu}^{2+}(aq) + 2text{e}^- rightarrow text{Cu}(s)$$
$$text{Overall: } text{Zn}(s) + text{Cu}^{2+}(aq) rightarrow text{Zn}^{2+}(aq) + text{
