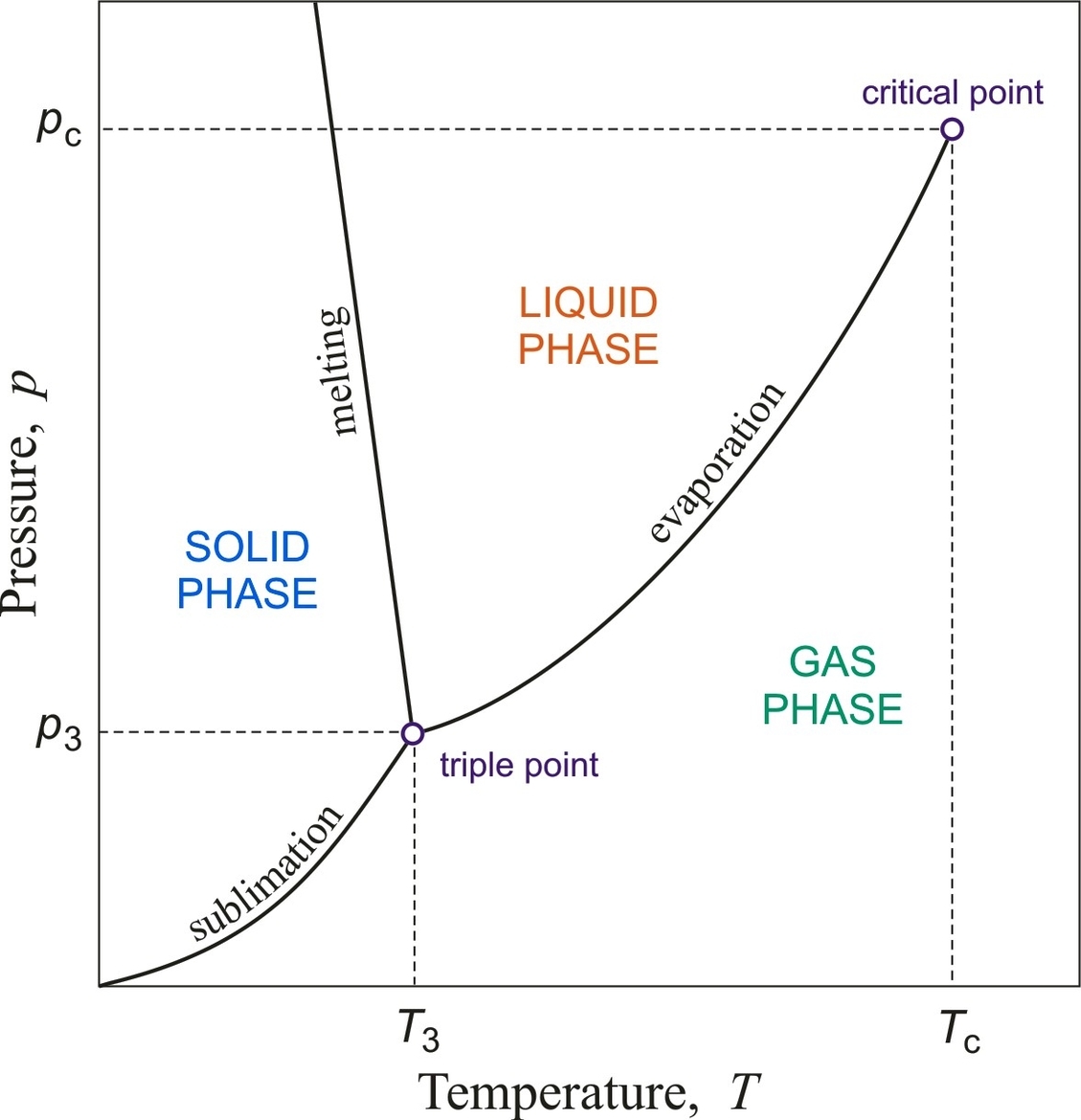
The critical point is a term used in thermodynamics to describe the end point of a phase equilibrium curve. It is the point at which two phases of a substance initially become indistinguishable from one another . One example of a critical point is the liquid-vapor critical point, which is the end point of the pressure-temperature curve that designates conditions under which a liquid and its vapor can coexist . At higher temperatures, the gas cannot be liquefied by pressure alone. At the critical point, defined by a critical temperature Tc and a critical pressure pc, phase boundaries vanish . Other examples include the liquid-liquid critical points in mixtures, and the ferromagnet-paramagnet transition (Curie temperature) in the absence of an external magnetic field .
The critical point is a unique condition that a substance must reach in order to undergo a phase transition. It is the point at which the distinction between different phases of a substance becomes blurred, and the substance exhibits unique properties . In the vicinity of the critical point, the physical properties of the liquid and the vapor change dramatically, with both phases becoming even more similar . For instance, liquid water under normal conditions is nearly incompressible, has a low thermal expansion coefficient, has a high dielectric constant, and is an excellent solvent for electrolytes. Near the critical point, all these properties change into the exact opposite: water becomes compressible, expandable, a poor dielectric, a bad solvent for electrolytes, and mixes more readily with nonpolar gases and organic molecules .
The critical point is a fundamental concept in thermodynamics and has many practical applications. For example, it is used in the design of power plants, refrigeration systems, and chemical processes . The critical point is also important in the study of phase transitions and critical phenomena, which are of great interest in condensed matter physics, materials science, and other fields .
In summary, the critical point is a unique condition that a substance must reach in order to undergo a phase transition. It is the point at which the distinction between different phases of a substance becomes blurred, and the substance exhibits unique properties. The critical point is a fundamental concept in thermodynamics and has many practical applications. It is also important in the study of phase transitions and critical phenomena, which are of great interest in condensed matter physics, materials science, and other fields..
