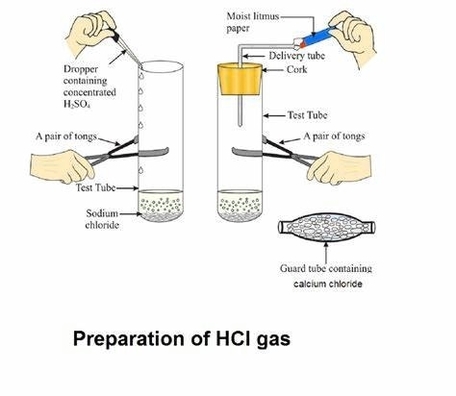
Acids, bases and salts are three classes of chemical compounds that have important roles in chemistry and everyday life. Here is a brief introduction to each of them.
Acids are substances that produce hydrogen ions (H+) or hydronium ions (H3O+) when dissolved in water. They have a sour taste, turn blue litmus paper red, and react with metals to produce hydrogen gas. Some common examples of acids are hydrochloric acid (HCl), sulfuric acid (H2SO4), and citric acid (C6H8O7).
Bases are substances that produce hydroxide ions (OH-) when dissolved in water. They have a bitter taste, turn red litmus paper blue, and feel slippery to the touch. Some common examples of bases are sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH3).
alts are ionic compounds that result from the neutralization reaction of an acid and a base. They usually consist of a metal cation and a nonmetal anion. They are neutral in nature, meaning they do not affect the color of litmus paper. Some common examples of salts are sodium chloride (NaCl), potassium nitrate (KNO3), and calcium carbonate (CaCO3).
The following table summarizes some of the properties and examples of acids, bases and salts.
| Property | Acid | Base | Salt |
| — | — | — | — |
| Taste | Sour | Bitter | None |
| Litmus paper | Red | Blue | No change |
| Reaction with metal | Hydrogen gas | No reaction | No reaction |
| Reaction with base | Salt and water | No reaction | No reaction |
| Reaction with acid | No reaction | Salt and water | No reaction |
| Example | HCl | NaOH | NaCl |
The strength of an acid or a base depends on how well it dissociates in water. Strong acids and bases completely dissociate into ions, while weak acids and bases partially dissociate. The pH scale is a measure of the acidity or alkalinity of a solution, ranging from 0 to 14. A solution with a
