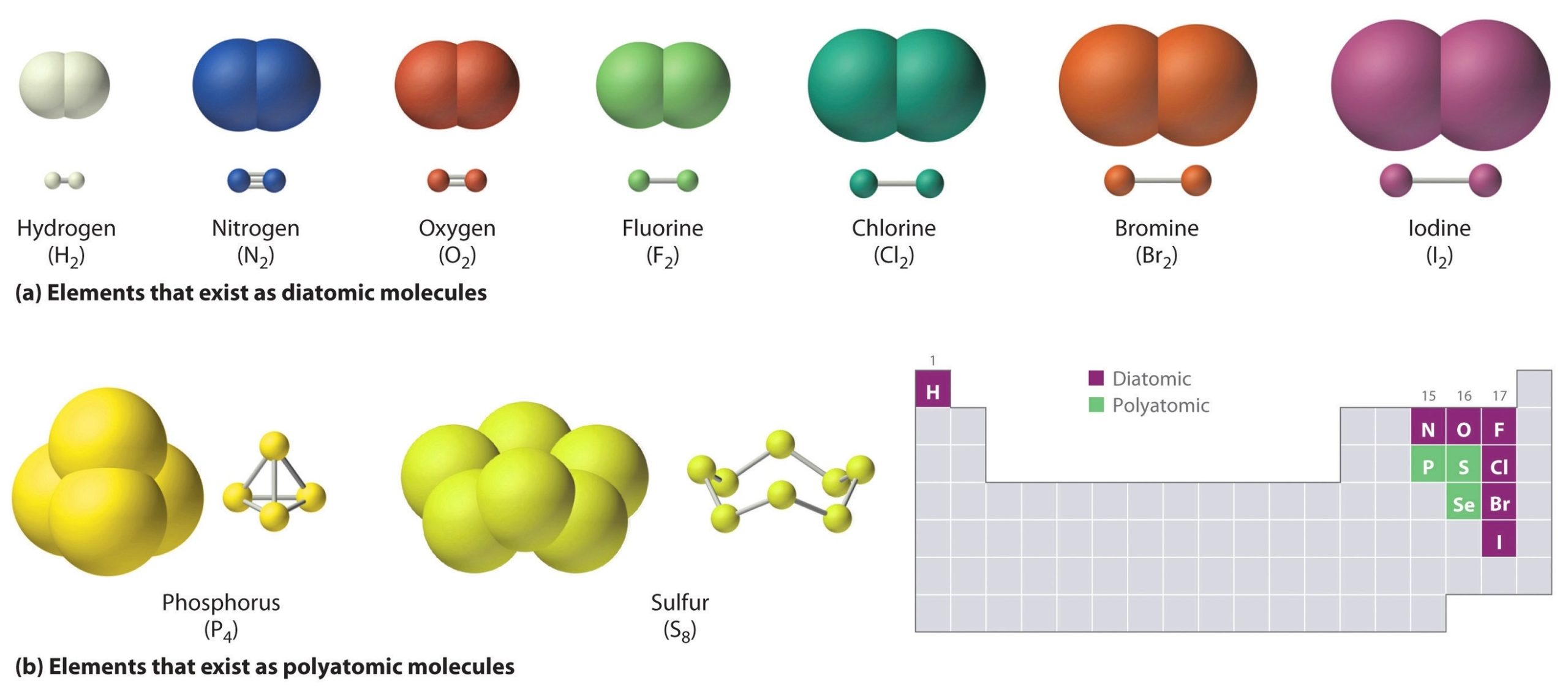
Covalent compounds are a type of chemical compound that are formed by the sharing of electrons between two or more non-metal atoms. This sharing of electrons is known as a covalent bond. Covalent bonds are formed when the electronegativity difference between two atoms is small, meaning that neither atom has a strong tendency to attract electrons. As a result, the atoms share electrons in order to achieve a more stable electron configuration.
Covalent compounds are also known as molecular compounds because they exist as discrete molecules. These molecules are held together by the covalent bonds between the atoms. The properties of covalent compounds depend on the strength of these bonds and the size of the molecules. Covalent compounds tend to have lower melting and boiling points than ionic compounds because the intermolecular forces between the molecules are weaker than the ionic bonds between ions in an ionic compound.
The naming of covalent compounds is based on the number of atoms of each element in the molecule. The prefixes used in the names of covalent compounds indicate the number of atoms of each element in the molecule. For example, CO2 is called carbon dioxide because it contains one carbon atom and two oxygen atoms. Similarly, N2O is called dinitrogen monoxide because it contains two nitrogen atoms and one oxygen atom.
Covalent compounds can be found in many different forms in nature. For example, water (H2O) is a covalent compound that is essential for life. Other examples of covalent compounds include methane (CH4), ammonia (NH3), and carbon dioxide (CO2). Covalent compounds are also used in many industrial applications, such as the production of plastics, synthetic fibers, and pharmaceuticals.
In summary, covalent compounds are formed by the sharing of electrons between two or more non-metal atoms. They exist as discrete molecules held together by covalent bonds. The properties of covalent compounds depend on the strength of these bonds and the size of the molecules. Covalent compounds can be found in many different forms in nature and are used in many industrial applications..
