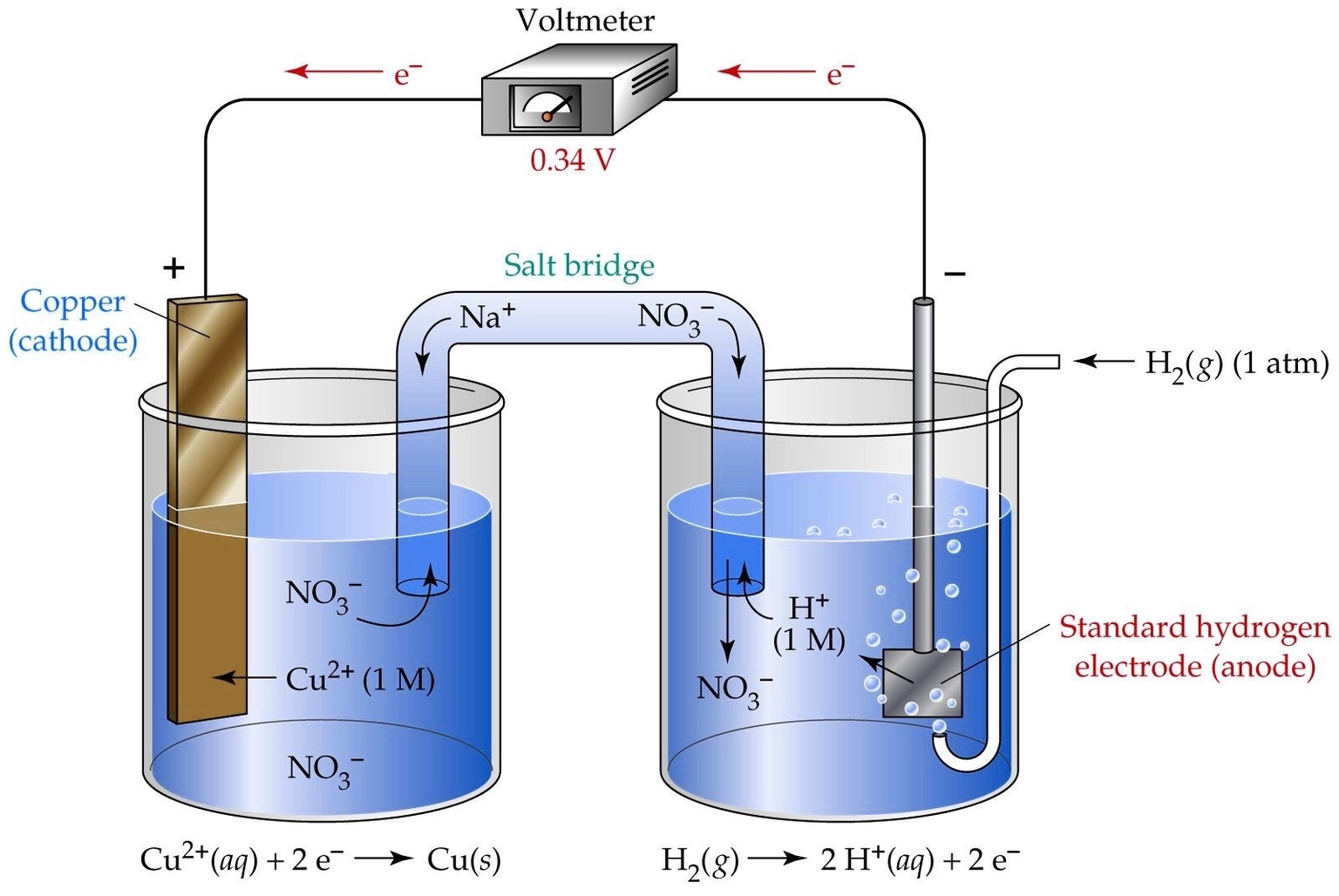
An electrochemical cell is a device that can either generate electrical energy from chemical reactions or use electrical energy to cause chemical reactions. There are two main types of electrochemical cells: galvanic cells and electrolytic cells. Galvanic cells produce electricity from spontaneous redox reactions, while electrolytic cells consume electricity to drive nonspontaneous redox reactions. Both types of cells consist of two half-cells, each containing an electrode and an electrolyte. The electrodes are connected by a wire, and the electrolytes are connected by a salt bridge or a porous membrane. The flow of electrons in the wire and the flow of ions in the salt bridge or membrane allow the redox reaction to occur in a controlled manner.
Galvanic cells are also known as voltaic cells, named after Alessandro Volta, who invented the first battery using a series of galvanic cells. A common example of a galvanic cell is the Daniell cell, which uses zinc and copper electrodes immersed in zinc sulfate and copper sulfate solutions, respectively. The zinc electrode is the anode, where oxidation occurs, and the copper electrode is the cathode, where reduction occurs. The overall reaction is:
$$Zn(s) + Cu^{2+}(aq) rightarrow Zn^{2+}(aq) + Cu(s)$$
The cell potential, or the voltage produced by the cell, depends on the difference in the standard reduction potentials of the two half-reactions. The standard reduction potential is a measure of how easily a substance gains electrons. The more positive the standard reduction potential, the more likely the substance is to be reduced. The cell potential can be calculated using the Nernst equation, which takes into account the concentrations of the reactants and products. The cell potential is also affected by the temperature and pressure of the system.
Electrolytic cells are the opposite of galvanic cells. They use an external source of electricity, such as a battery or a power supply, to force a nonspontaneous redox reaction to occur. Electrolytic cells are often used for electroplating, which is the process of coating a metal object with another metal using electricity. For example, an electrolytic cell can be used to plate a copper object with silver. The copper object is connected to the negative terminal of the power supply, making it the cathode, where reduction occurs. A
